Customer Support
Product Orders
(866) 246-6990 USA Product orders by FAX: (866) 804-9881 USA Email Customer Service Contact Us Form

Arrow-Clark™ VectorFlow® Chronic Hemodialysis Catheter
Because in chronic hemodialysis catheters, the tip matters
The Arrow-Clark™ VectorFlow® Catheter is a symmetrical-tip, tunneled hemodialysis catheter designed to provide sustained high flows,1 reduce loss of lock solution2 and reduce the risk of thrombus accumulation due to platelet activation resulting from shear stress.3 Available in both retrograde and antegrade insertion platforms, the catheter is designed with an innovative tip that allows placement flexibility with minimal impact on recirculation rates.2
Arrow-Clark™ VectorFlow® Chronic Hemodialysis Catheter Animation
Arrow-Clark™ VectorFlow® Chronic Hemodialysis Catheter Antegrade Insertion
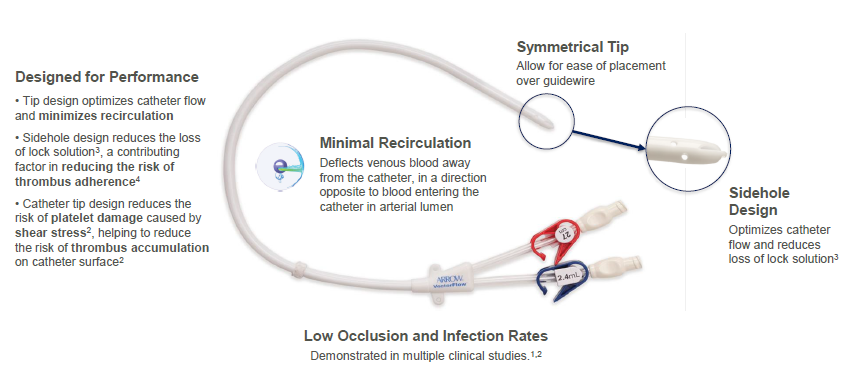
Designed for ease of placement
- No stylet needed for insertion
- Available in antegrade and retrograde insertion platforms
- Catheter tip designed to allow for placement flexibility with minimal impact on recirculation rates2
References:
- Ross JR, Puga TA, & Philbeck TE. Longitudinal dialysis adequacy and clinical performance of the VectorFlow hemodialysis catheter: a prospective study. J Vasc Access. 2017;18(6):492-497. Study sponsored by Teleflex. Ross JR was formerly a paid consultant of Teleflex. Puga TA and Philbeck TE are employees of Teleflex.
- Data on file at Teleflex. Test data may not be indicative of clinical performance.
- Nobili M, Sheriff J, Morbiducci U, et al. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in-vitro measurements. ASAIO J. 2008;54(1):64-72.
- Data on file at Teleflex. Test data may not be indicative of clinical performance.
- Clark TWI, Isu G, Gallo D, et al. Comparison of symmetric hemodialysis catheters using computational fluid dynamics. J Vasc Interv Radiol. 2015;26(2):252-259. Data on file at Teleflex. Computer modeling may not be indicative of clinical performance. Timothy Clark, MD, is a paid consultant and receives royalty payments from Teleflex or its affiliates for the Arrow-Clark VectorFlow Chronic Hemodialysis Catheter.
- National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations. Guideline 7: Prevention and treatment of catheter and port complications. 2006 update. Am J Kidney Dis. 2006;48:1-322.
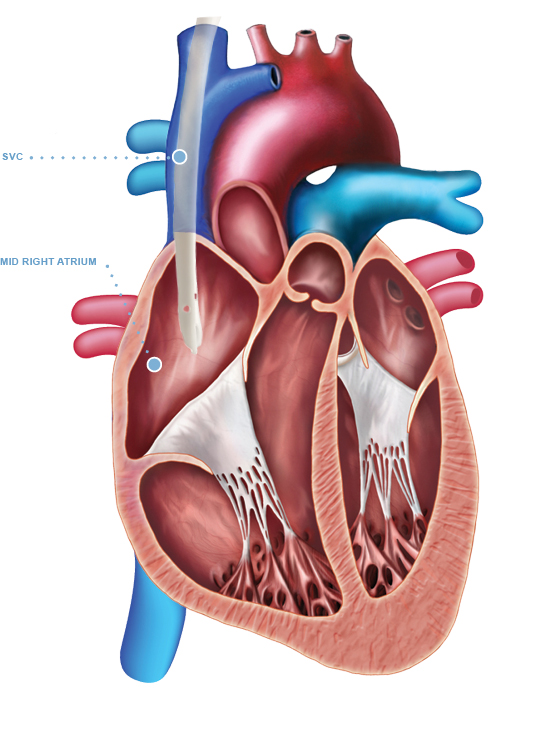
The Arrow-Clark™ VectorFlow® Retrograde Catheter is indicated for use in attaining long-term vascular access for hemodialysis and apheresis. The Arrow-Clark™ VectorFlow® Retrograde Catheter is inserted percutaneously and is preferentially placed into the internal jugular (IJ) vein. Alternately, this catheter may be inserted into the subclavian vein although the jugular vein is the preferred site. Catheters greater than 40 cm are intended for femoral vein insertion. The Arrow-Clark™ VectorFlow® Retrograde Catheter is intended for use in adult patients.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Not all products are available in all regions. Please contact customer service to confirm availability in your region.
VectorFlow is a registered trademark of Aegis Medical Technologies LLC.
Teleflex, the Teleflex logo and Arrow-Clarke are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-004319 Rev 2
Revised: 09/2021.