Customer Support
Product Orders
(866) 246-6990 USA Product orders by FAX: (866) 804-9881 USA Email Customer Service Contact Us Form
USA /
Product Areas /
Interventional Cardiology/Radiology /
Drainage /
Arrow™ Diagnostic & Drainage Products

Arrow® Diagnostic and Drainage Products
Arrow® Diagnostic and Drainage Products save time when it matters most1
In trauma situations, you need fast, effective solutions at your fingertips. Arrow® Diagnostic and Drainage Products are designed to let you perform a variety of procedures with ease and efficiency, to save you critical seconds when needed most.
We offer you a range of options for performing your diagnostic and drainage procedures.
- Arrow® Pneumothorax Kit for percutaneous aspiration of simple or spontaneous pneumothorax
- Arrow® Large Volume Paracentesis Kit
- Arrow-Clarke™ Thoracentesis Catheter with Pleura-Seal® Valve
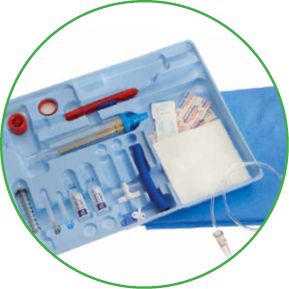
- Arrow® Cavity Drainage Catheterization Kit offers a choice of techniques with exclusive Blue FlexTip® Drainage Catheters and advanced internal tip design to facilitate placement
References:
- A New Instrument for Thoracentesis, John M. Clarke, M.D., F.A.C.S., St. Petersburg, Florida; Surgery, Gynecology & Obstetrics, December 1984, Volume 159.
The Arrow® Pneumothorax Product facilitates
lung reexpansion of
a minor pneumothorax.
The Arrow® Large Volume Paracentesis Kit permits access to the abdominal cavity for drainage and specimen collection.
The Arrow-Clarke™ Thoracentesis Device permits the removal of fluids from the pleural space and provides access to infuse chemotherapeutic or sclerosing agents.
The Cavity Drainage Catheterization Product permits percutaneous placement of a catheter into a cavity for drainage.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Not all products are available in all regions. Please contact customer service to confirm availability in your region.
Teleflex, the Teleflex logo Teleflex, Arrow, Arrow-Clarke, Blue FlexTip and Pleura-Seal are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-004198 Rev 1
Revised: 01/2021.
The Arrow® Large Volume Paracentesis Kit permits access to the abdominal cavity for drainage and specimen collection.
The Arrow-Clarke™ Thoracentesis Device permits the removal of fluids from the pleural space and provides access to infuse chemotherapeutic or sclerosing agents.
The Cavity Drainage Catheterization Product permits percutaneous placement of a catheter into a cavity for drainage.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Not all products are available in all regions. Please contact customer service to confirm availability in your region.
Teleflex, the Teleflex logo Teleflex, Arrow, Arrow-Clarke, Blue FlexTip and Pleura-Seal are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-004198 Rev 1
Revised: 01/2021.