Customer Support
Product Orders
(866) 246-6990 USA Product orders by FAX: (866) 804-9881 USA Email Customer Service Contact Us Form
USA /
Product Areas /
Interventional Cardiology/Radiology /
Coronary Interventions /
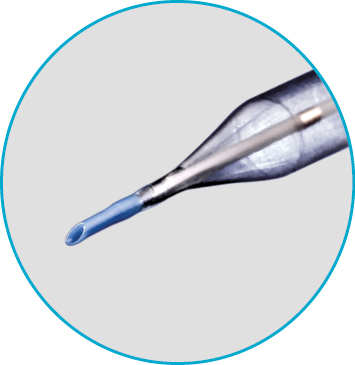
Glider™ PTCA Balloon Catheter

Glider™ PTCA Balloon Catheter
The Glider™ PTCA Balloon Catheter is a semi-compliant balloon with skived tip and low entry profile. The tip design, coupled with hydrophilic coating and an innovative, torqueable shaft, allows for precise tip orientation for use in crossing complex lesions and stent struts.
- Low-profile crossability
- Demonstrated clinical performance in crossing stent struts and bifurcations1
- Skived tip design, hydrophilic coating, and torqueable shaft allow for precise tip orientation
References:
- Secco, G. G., Rittger, H., Hoffmann, S., Richardt, G., Abdel-Wahab, M., Reinecke, H., ... Mario, C. D. (2016). The Glider Registry: A Prospective Multicenter Registry of a new Ultrashort Dedicated Balloon for Side-Branch Ostial Dilatation. Catheterization and Cardiovascular Interventions, 89(1). doi:10.1002/ccd.25040.
The Glider™ PTCA Balloon Dilatation Catheter
is indicated for
balloon dilatation of the stenotic portion of coronary artery or bypass graft
stenosis for the
purpose of improving myocardial perfusion.
The Glider™ PTCA Balloon Dilatation Catheter is contraindicated for unprotected left main coronary artery and coronary artery spasm in the absence of a significant stenosis.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. Information in this material is not a substitute for the product Instructions for Use.
Not all products are available in all regions. Please contact customer service to confirm availability in your region.
Teleflex, the Teleflex logo and Glider are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-004656 Rev 0
Revised: 10/2018.
The Glider™ PTCA Balloon Dilatation Catheter is contraindicated for unprotected left main coronary artery and coronary artery spasm in the absence of a significant stenosis.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. Information in this material is not a substitute for the product Instructions for Use.
Not all products are available in all regions. Please contact customer service to confirm availability in your region.
Teleflex, the Teleflex logo and Glider are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-004656 Rev 0
Revised: 10/2018.