Product Features
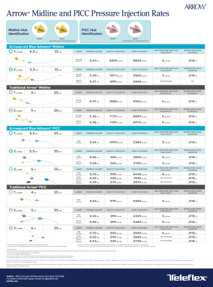
Pressure injection
Up to 5mL/second through the distal lumen
Up to 5mL/second through the distal lumen
Single and dual lumen design options available
Antimicrobial/antithrombogenic
Chlorhexidine-based technology provides protection on the external catheter surface from tip to juncture hub and the entire intraluminal pathway including the luer hub and extension lines2,3
Chlorhexidine-based technology provides protection on the external catheter surface from tip to juncture hub and the entire intraluminal pathway including the luer hub and extension lines2,3
Staggered exit ports
Reduces risk of mixing incompatible drugs and solutions that may create precipitate4
Reduces risk of mixing incompatible drugs and solutions that may create precipitate4
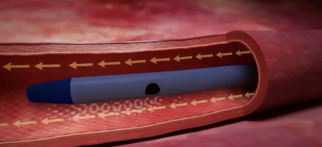
Blue FlexTip® Catheter
Proprietary design minimizes risk of vessel damage1
Proprietary design minimizes risk of vessel damage1
Bright yellow pinch clamps and hubs for easy midline identification.
Additional Resources
Arrow™ Midline Catheters + Arrowg+ard Blue Advance™ Midline Catheter Brochure
PICC Catheters - Tapered vs. Non-Tapered
Arrow™ Pressure Injectable Midline Brochure
Arrow™ Midline and PICC Pressure Injection Rates
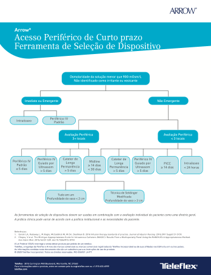
Short-term Peripheral Access Device Selection Tool
Arrowg+ard Blue Advance™ Midline Catheters Brochure