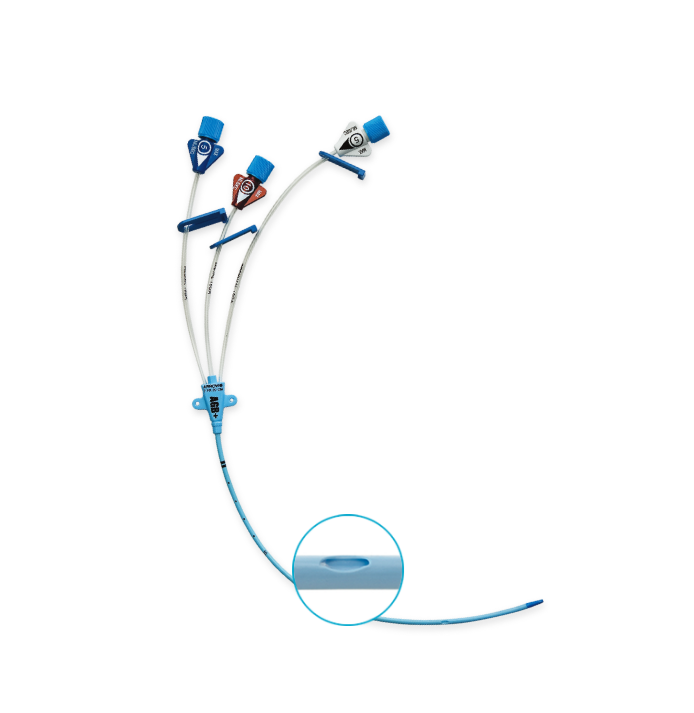
Arrowg+ard Blue Plus® CVC
Central Venous Catheters
Arrow® Arrowg+ard Blue Plus® CVC is the only full-spectrum antimicrobial CVC that protects against:
- Gram-positive bacteria
- Gram-negative bacteria
- Fungi
REQUEST A DEMO





|

|

|

|
|---|---|---|---|
|
10 mL/second Maximum flow rate. |
Pressure injection information marked clearly on the hubs. |
Staggered Exit Ports
Reduces the risk of mixing incompatible drugs and solutions that may create precipitate.1 |
Blue FlexTip® Feature
Soft, contoured tip design minimizes the risk of vessel. |
Arrowg+ard Blue Advance™
PICC
The PICC with Antimicrobial and Antithrombogenic Protection.3,4 The Arrowg+ard Blue Advance™ PICC is designed to protect both internal and external catheter surfaces with chlorhexidine, reducing the risk of these catheter-related complications:
- Intraluminal thrombotic occlusion2,3,4,5
- Microbial colonization1,3
- Phlebitis4,6
- Intimal hyperplasia4,6
REQUEST A DEMO






|

|

|

|
Arrowg+ard Blue Advance™ Protection
Chemically bonded to the catheter
surface, providing intraluminal and
extraluminal antimicrobial and
antithrombogenic protection
of the catheter surface for at least |
TaperFree™ Catheter
May minimize risk of catheter-related thrombosis and ensures stated French size is consistent between distal and proximal ends.7,8,9 |
Pressure Injectable |
Staggered Exit Ports
Reduces the risk of mixing incompatible drugs and solutions that may create precipitate.1,6 |
|---|
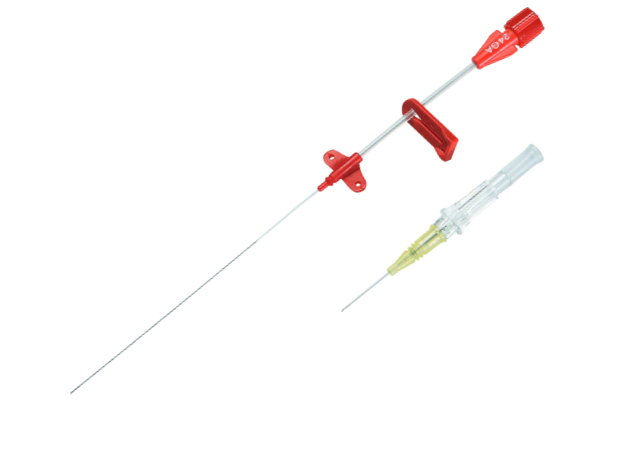
Arrow® Seldinger Arterial Catheter
The Arrow® Seldinger Arterial Catheter is designed to improve patient safety by eliminating confusion of catheter identification, reduce complications associated with insertion technique, and provide optimal performance leading to effective treatment for patients. Available in multiple sizes to provide customization to each patient, each insertion site, and each application.
REQUEST A DEMO




|

|

|
|---|---|---|
|
Designed for Patient Safety
Minimizes trauma to vessel wall and eliminates confusion of catheter identification. |
Reduces Complications:
Designed for less vessel movement, irritation, infiltration, and accidental disconnections. |
Advanced Performance:
Innovative catheter material demonstrated high resistance to collapse, low thrombus accumulation and occlusion10 |
Gibeck® Humid-Flo® HME
The exclusive design of the Gibeck Humid-Flo HME allows it to remain in-line during the first 72 hours of mechanical ventilation, even during aerosol treatments.
REQUEST A DEMO






|

|

|

|
|---|---|---|---|
|
Reduces circuit breaks aiding clinician adherence to clinical guidelines regarding infection control practices |
Protects caregivers from exposure to depressurizing circuit spray |
Hygroscopic bacteriostatic microwell paper media provide optimal moisture output with low resistance to flow |
Reduces the need to routinely interrupt ventilation, therefore maintains positive-end expiratory pressure |
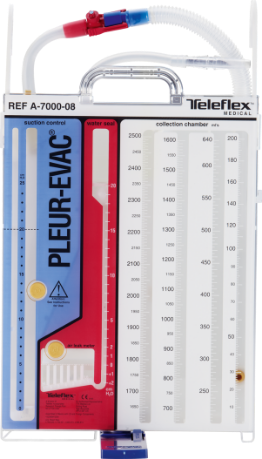
Pleur-evac®
Chest Drainage Systems
The premiere solution for thoracic cardiovascular, trauma, and critical care - uses the most advanced fluid management technology available.
REQUEST A DEMO






|

|

|

|

|
|---|---|---|---|---|
|
Accurate, calibrated, high suction control with visual indicator. |
Clinically supported patient air leak meter. |
Complete positive and negative pressure controls. |
Latex-free tubing available on all Pleur-evac models |
Generous collection chambers holding up to 2500 cc's of fluid. |