MANTA® Vascular Closure Device
The MANTA® Device is the first commercially available biomechanical vascular closure device designed specifically for large bore femoral arterial access site closure.1 Available in 14 Fr. and 18 Fr., a single MANTA® Device effectively closes femoral arterial access sites following the use of sheaths ranging from 12 Fr. to 25 Fr. O.D.2a
Simple Deployment
Addresses the challenges of large bore closure with a single easy-to-use device.2a
Rapid Hemostasis
Reduces time to hemostasis without pre-closure, utilizing the coagulation-inducing properties of
collagen for rapid hemostasis to promote vessel healing.2c,3-5
Reliable Closure
Delivers reproducible results and helps inspire confidence in achieving successful
closure.2b

Clinically Proven
The SAFE MANTA IDE Clinical Trial, the largest U.S. prospective, multi-center study of a purpose-designed large bore femoral arterial access site closure device to date, demonstrated the safety and effectiveness of the MANTA® Device with all primary and secondary endpoints met.2
Product Features
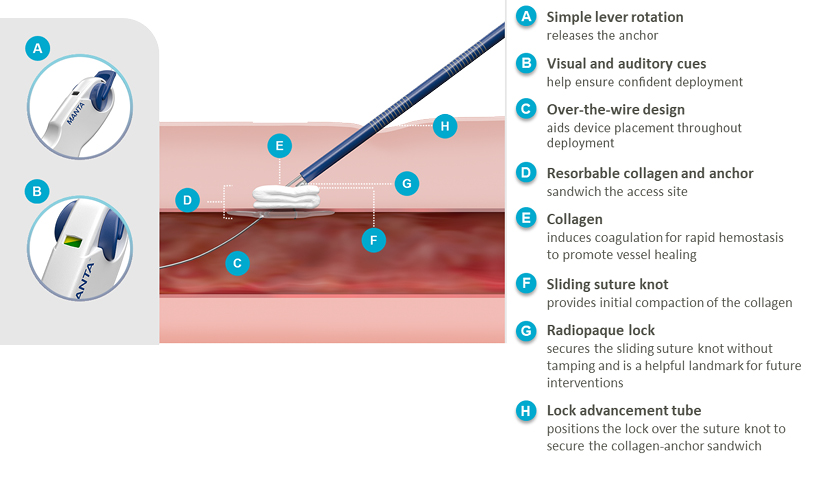
MANTA® Features & Benefits
MANTA® Device Deployment
References:
- Data on file at Teleflex.
- Data on file at Teleflex. The SAFE MANTA IDE Clinical Trial.
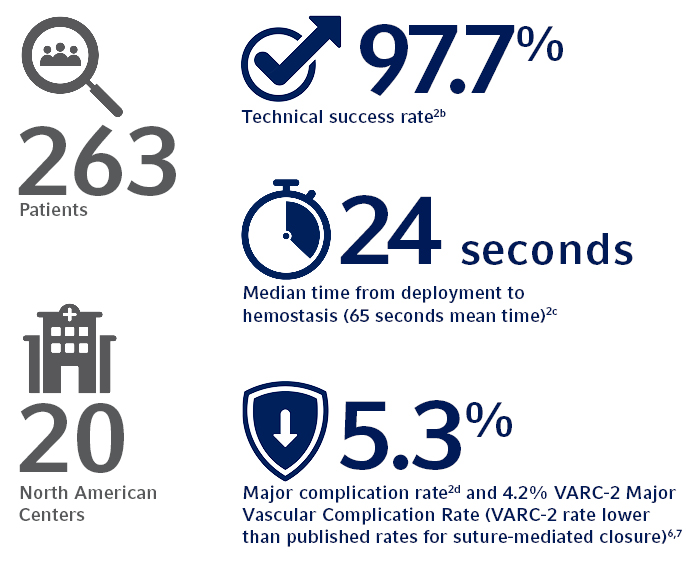
a. A single MANTA™ Vascular Closure Device was deployed in 99.6% of subjects in IDE trial.
b. 97.7% Technical Success, defined as percutaneous vascular closure obtained with the MANTA™ Device without the use of unplanned endovascular or surgical intervention.
c. The MANTA™ Device demonstrated a time to hemostasis (TTH) of 24 seconds median time (65 seconds mean time) from deployment to hemostasis, which is lower than published rates for Perclose ProGlide® where Perclose ProGlide® demonstrated a TTH of 9.8 +/- 17 minutes (588 +/- 1,020 seconds).3
d. Major Complications defined as composite of: i) vascular injury requiring surgical repair/stent-graft; ii) bleeding requiring transfusion; iii) lower extremity ischemia requiring surgical repair/additional percutaneous intervention; iv) nerve injury (permanent or requiring surgical repair); and v) infection requiring IV antibiotics and/or extended hospitalization.
Study sponsored by Teleflex Incorporated or its affiliates. - Nelson PR, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. 2014 May;59(5):1081-1193.
- Farndale RW, Sixma JJ, Barnes MJ, de Groot, PG. The role of collagen in thrombosis and hemostasis, J Thromb Haemost. 2004 Apr,2(4);564-573.
- Nuyttens BP, Thijs T, Deckmyn H, Broos K. Platelet adhesion to collagen, Thromb Res. 2011;127(2); S26-S29.
- Généreux P, et al. Vascular complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2012 Sept 18;60(12):1043-1052.
- Lauten A, et al. Percutaneous left-ventricular support with the Impella 2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013 Jan;6(1):23-30.
CONTRAINDICATIONS: There are no known contraindications to the use of this device.
WARNINGS: 1) Do not use if the puncture site is proximal to the inguinal ligament or above the most inferior border of the epigastric artery (IEA), as this may result in retroperitoneal bleeding. 2) Do not use in patients with severe calcification of the access vessel and/or common femoral artery stenosis resulting in a vessel <5mm in diameter for the 14F MANTA or <6mm in diameter for the 18F MANTA, or>50% diameter femoral or iliac artery stenosis. 3) Do not use in patients with severe peripheral vascular disease, as evidenced by severe claudication when ambulating <100 feet, weak or absent pulses in the affected limb, or ABI <0.5 at rest. 4) Do not use if the temperature indicator dot on package has changed from light gray to dark gray or black. 5) Do not use if the package is damaged or any portion of the package has been previously opened. 6) Do not use if the items in the package appear damaged or defective in any way. 7) Do not REUSE or RESTERILIZE. The MANTA Device is single use only. The MANTA Device contains bioresorbable materials that cannot be reused or re-sterilized. Reuse or re-sterilization may cause degradation to the integrity of the device, leading to device failure which may result in patient injury, illness, or death. 8) Do not use the MANTA Device where bacterial contamination of the procedure sheath or surrounding tissues may have occurred, as this may result in infection. 9) Do not use if the MANTA delivery system becomes kinked. 11) Do not inflate a contralateral balloon in the femoral or iliac artery during MANTA Sheath exchange or the MANTA Closure procedure. 12) Do not use MANTA if there has been a femoral artery puncture in same vessel within the prior 30 days, recent femoral artery puncture in same groin that has not healed appropriately, and/or recent (<30 days) vascular closure device placement in same femoral artery. 13) Do not use if the puncture site is at or distal to the bifurcation of the superficial femoral and profunda femoris artery, as this may result in the (a) anchor catching on the bifurcation or being positioned incorrectly, and/or (b) collagen deposition into the vessel. 14) Do not use if there is difficult dilation from initial femoral artery access (e.g., damaging or kinking dilators) while step dilating up to the large-bore device. Difficult dilation of the puncture tract due to scar tissue may lead to swelling of surrounding tissue, thus compromising the accuracy of the puncture depth determined during the puncture location procedure. 15) Do not use if sheath insertion is in a vessel other than the femoral artery. 16) Do not use if there is marked tortuosity of the femoral or iliac artery. 17) Do not use if the patient has marked obesity or cachexia (BMI>40 kg/m2 or <20 kg/m2). 18) Do not use if the patient has post-procedure blood pressure>180 mmHg that cannot be lowered prior to access site closure. 19) Do not use in patients who cannot be adequately anticoagulated for the procedure. 20) Do not use the MANTA Device in patients with known allergies to bovine products, collagen and/or collagen products, polyglycolic or polylactic acid polymers, stainless steel or nickel.
PRECAUTIONS: 1) The MANTA Device should only be used by a licensed physician or healthcare provider trained in the use of this device. 2) This device contains a small radiopaque stainless-steel lock that is implanted in the puncture tract. See MRI information in these instructions for use and patient implant card. 3) In the event that bleeding from the femoral access site persists after the use of the MANTA Device, the physician should assess the situation. Based on the physician assessment of the amount of bleeding, use manual or mechanical compression, application of balloon pressure from a secondary access site, placement of a covered stent, and/or surgical repair to obtain hemostasis.
POTENTIAL ADVERSE EVENTS: The following potential adverse events related to the deployment of Vascular Closure Devices have been identified: 1) Ischemia of the leg or stenosis of the femoral artery. 2) Local trauma to the femoral or iliac artery wall, such as dissection. 3) Retroperitoneal bleeding as a result of access above the inguinal ligament or the most inferior border of the epigastric artery (IEA). 4) Perforation of iliofemoral arteries, causing bleeding/hemorrhage. 5) Thrombosis formation or embolism. 6) Nerve damage or neuropathy. 7) Other access site complications leading to bleeding, hematoma, pseudoaneurysm, or arterio-venous fistula, possibly requiring blood transfusion, surgical repair, and/or endovascular intervention. Potential Adverse Events associated with any large bore intervention, including the use of the MANTA Vascular Closure Device, include but are not limited to: Arterial damage; Arterio-venous fistula; Bradycardia; Compartment syndrome; Death related to the procedure; Deep vein thrombosis; Ecchymosis; Edema; Infection at the puncture site which may require antibiotics or extended hospitalization; Inflammatory response; Late arterial bleeding; Oozing from the puncture site; Pressure in groin/access site region; Vessel laceration or trauma; Wound dehiscence.
Please see the instructions for use for complete product information.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Not all products are available in all regions. Please contact customer service to confirm availability in your region.
Teleflex, the Teleflex logo, and MANTA are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. All other trademarks or registered trademarks are property of their respective owners.
MC-005922 LA ES Rev 0 Revised: 09/2019.