
D-Stat® Rad-Band Topical Hemostat
Utilizing the power of thrombin
The D-Stat® Rad-Band Topical Hemostat contains the power of thrombin in a familiar D-Stat® Dry Topical Hemostat pad. The device includes an easy release clasp for single-operator pressure adjustments, and adjustable foam pads for additional patient comfort.
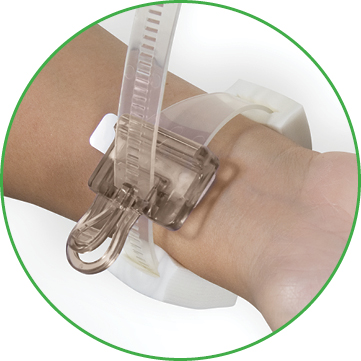
The D-Stat® Rad-Band is contraindicated in persons with known sensitivity to bovine-derived materials.
WARNING: SEVERE
BLEEDING AND THROMBOSIS COMPLICATIONS
- THROMBIN-JMI® can cause fatal severe bleeding or
thrombosis. Thrombosis may
result from the development of antibodies against bovine thrombin.
Bleeding may result from the
development of antibodies against factor V. These may cross-react
with human factor V and lead
to its deficiency.
- Do not re-expose patients to THROMBIN-JMI® if there
are known or suspected
antibodies to bovine thrombin and/or factor V.
- Monitor patients for abnormal coagulation laboratory values,
bleeding, or thrombosis.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
THROMBIN-JMI is a registered trademark of King Pharmaceuticals Research and Development, LLC.
Teleflex, the Teleflex logo and D-Stat are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-005114