
D-Stat® Flowable Hemostat
Shown to reduce the frequency of clinically relevant hematoma formation in pulse generator implants1,2,3
Comprised of collagen, thrombin and a buffered diluent, this thick, yet flowable procoagulant facilitates hemostasis by initiating the body’s own clotting mechanisms.
Practical advantages
- Fully sterile kit allows complete preparation within sterile field
- Easy, needle-free mixing
- No pharmacy storage required
The Pocket Protector Study showed a 48% reduction in clinically relevant hematomas1,2,3
The Pocket Protector Study was a prospective, randomized clinical study evaluating the incidence of clinically relevant hematoma formation after pulse generator implants using D-Stat® Flowable Hemostat plus standard of care vs. standard of care alone.4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*Statistically significant reduction (p ≤ 0.05) calculated using Fischer’s Exact Test. |
|||
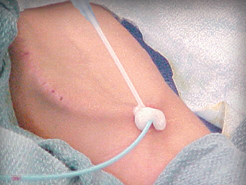
D-Stat® Flowable Hemostat for use in vascular access sites
Applied topically to control bleeding from vascular access sites and percutaneous catheters and tubes.
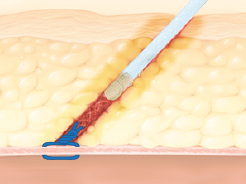
D-Stat® Flowable Hemostat for use in tissue tract hemostasis post vascular closure device
Applied as an adjunct treatment to seal residual oozing of tissue tracts of femoral access sites that have been previously closed by suture/collagen-based hemostatic devices.
References:
- Slotwiner D, Alder S, Fuenzalida C, McCowan R, et al. The Pocket
Protector Study: Use of
D-Stat® Flowable Hemostat in Pulse Generator
Pectoral Pockets Reduces the Rate
of Clinically Relevant Hematomas. Circulation. 2007;116
:II_678 (the "Pocket Protector Study"). - The Pocket Protector Study evaluated 269 high-risk patients, which were defined as those whose anticoagulation regimens were resuming within 24 hours. These regimens included the administration of one or more of the following medications: Heparin, LMWH, Coumadin®, Plavix®. For patients receiving Coumadin therapy, an INR of < 2.0 was required.
- Shapes of hematomas vary depending on the tissue planes that are
dissected in. In the Pocket
Protectory Study the following formula was used to identify clinically relevant pocket hematomas. "Length, width, and height of any palpated hematoma to approximate the amount of blood present using the following formula for the volume of an ellipsoid: V = (1/6) π (L)(W)(H). Where: V = Volume, L = Length (cephalead /caudal), W = Width (medial/lateral), and H = Height (anterior to pulse generator)." "Based on this formula, the volume of blood present in a hematoma measuring 8 x 1 x 2 (cm) (L,W ,H ) is approximately 8.5ml, therefore this volume shall serve as the minimum volume for a clinically relevant hematoma." - The Pocket Protector Study defined standard of care as compression, electrocautery and/or untreated cotton pledgets.
D-Stat® Flowable Hemostat is indicated for use in high-risk, anti-coagulated patients undergoing implantation of a pulse generator (e.g., pacemaker or ICD) to reduce the frequency of clinically relevant hematoma formation in the prepectoral pocket. High-risk patients are defined as those whose anti-coagulation regimens will resume within 24 hours of implant. Clinically relevant hematomas are defined as those that result in an alteration in the standard of care resultant of hematoma formation including alteration (i.e. suspension or discontinuation) of the anticoagulant therapy regimen (Heparin, LMWH, Coumadin® or Plavix®), application of a compression bandage and evacuation of the hematoma.
The D-Stat® Flowable Hemostat is contraindicated in persons with known sensitivity to bovine-derived materials.
WARNING: SEVERE
BLEEDING AND THROMBOSIS COMPLICATIONS
- THROMBIN-JMI® can cause fatal severe bleeding or
thrombosis. Thrombosis may
result from the development of antibodies against bovine thrombin.
Bleeding may result from the
development of antibodies against factor V. These may cross-react
with human factor V and lead
to its deficiency.
- Do not re-expose patients to THROMBIN-JMI® if there
are known or suspected
antibodies to bovine thrombin and/or factor V.
- Monitor patients for abnormal coagulation laboratory values,
bleeding, or thrombosis.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Plavix is a registered trademark of Sanofi-Societe Anonyme.
Coumadin is a registered trademark of Bristol-Myers Squibb Pharma Company.
THROMBIN-JMI is a registered trademark of King Pharmaceuticals Research and Development, LLC.
Teleflex, the Teleflex logo and D-Stat are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-005114