Latin America /
Product Areas /
Interventional Cardiology/Radiology /
Hemodialysis /
Arrow Edge® Chronic Hemodialysis Catheter
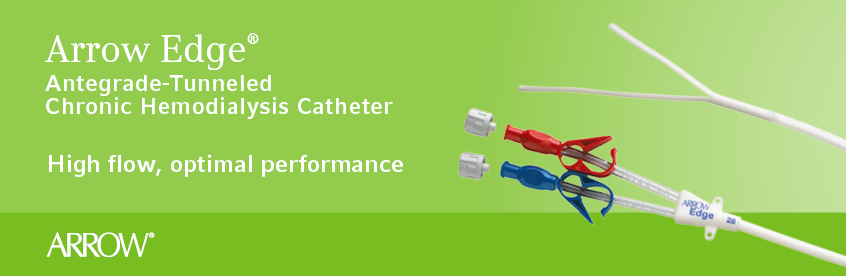
Arrow Edge® Antegrade-Tunneled Chronic Hemodialysis Catheter
Staggered V-Tip Design for Optimal Performance
The Arrow Edge® Catheter is designed to provide optimal performance by delivering high flow rates, up to 400 cc per minute, at low pressures.1 The crescent-shape of the catheter 's distal V- tip is designed to promote blood flow even if the tip is resting against the vessel wall.
- Innovative V-tip design and catheter tip orientation minimize the risk of recirculation when placed in the right atrium2
- Compatible with broad spectrum of antiseptic agents, including alcohol and iodine3
- Kits feature Arrow® SmartSeal® Hemostatic Peelable Dialysis Sheath
References:
- In vitro test performed by an independent laboratory (Citech, Plymouth Meeting, PA). Laboratory test results may not be indicative of clinical performance.
- Caridi J, Ross E, Aspilcueta A, Wiley S. The cannon-catheter-a prospective analysis. J of Vasc and Interv Radiol. 2010;21:1588-1590. The Arrow Edge® Catheter is the antegrade version of and has the same tip design as the retrograde Arrow® Cannon® Catheter. Both catheters are designed for placement in the mid-right atrium.
- Alcohol, alcohol-based solutions (e.g.,
Hibiclens®,
ChloraPrep®), iodine-based solutions
(Povidone-Iodine), PEG-based ointments
(e.g., Bactroban®), hydrogen peroxide or
ExSept Plus® are accepted for use with this catheter.
The Arrow Edge® catheter is indicated
for use in attaining
long-term vascular access for hemodialysis and apheresis. The Arrow
Edge® catheter
is inserted percutaneously and is preferentially placed into the
internal jugular (IJ) vein.
Alternately, this catheter may be inserted into the subclavian vein
although the jugular vein is
the preferred site. Catheters greater than 40 cm are intended for
femoral vein insertion. Arrow
Edge® catheter is intended for use in adult patients.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Hibiclens is a registered trademark of Regent Medical Limited.
ChloraPrep is a registered trademark of Carefusion 2200, Inc.
Bactroban is a registered trademark of GlaxoSmithKline, LLC.
ExSept Plus is a registered trademark of Angelini Pharma, Inc.
Teleflex, the Teleflex logo, Arrow, Arrow Edge and SmartSeal are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-005112
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Hibiclens is a registered trademark of Regent Medical Limited.
ChloraPrep is a registered trademark of Carefusion 2200, Inc.
Bactroban is a registered trademark of GlaxoSmithKline, LLC.
ExSept Plus is a registered trademark of Angelini Pharma, Inc.
Teleflex, the Teleflex logo, Arrow, Arrow Edge and SmartSeal are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-005112