
Arrow® Temporary Pacing Catheters and Kits
Arrow® Temporary Pacing Catheter Kit
When every minute matters
Reach for an Arrow® Temporary Pacing Catheter Kit from Teleflex, and you will discover everything you need to place a pacing catheter at the patient’s bedside, quickly and efficiently when time matters most. The Arrow® Pacing Kit provides clinicians with access to all components typically needed for insertion and components that are compatible with one another; enabling clinicians to start patient care sooner by eliminating the need to gather separate, yet necessary components for pacing insertion procedures.
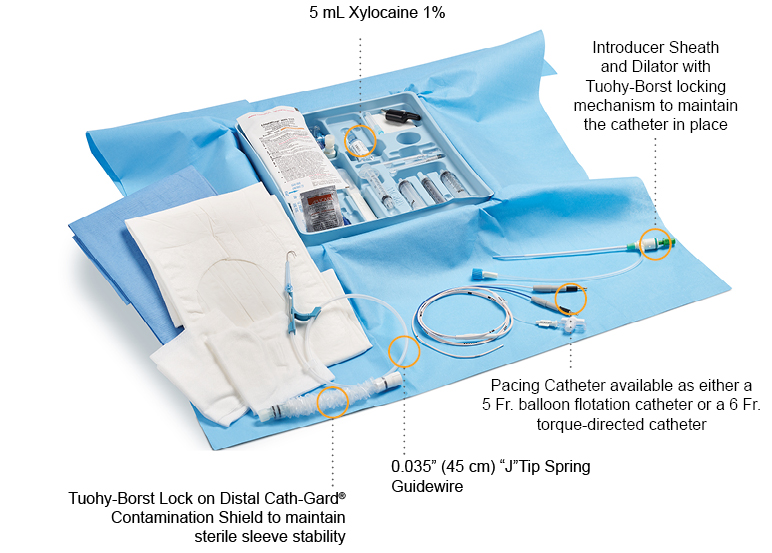
Arrow® Temporary Pacing Catheters
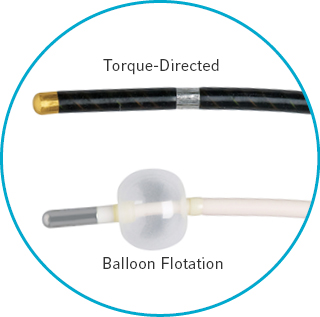
Two styles of bi-polar pacing catheters
- Torque-directed catheter provides excellent maneuverability and mechanical stability
- Balloon flotation catheters allow easy positioning at the bedside without fluoroscopy
The Arrow® Balloon Temporary Pacing Catheter is indicated for use in temporary transvenous ventricular cardiac pacing when impulse formation or conduction is impaired.
The Arrow® Bipolar Temporary Pacing Catheter with stylet is indicated for use in temporary transvenous cardiac pacing when impulse formation or conduction is impaired.
The Arrow® Percutaneous Sheath Introducer permits venous access and catheter introduction to the central circulation.
The Arrow® Sheath Adapter is utilized in conjunction with one-piece Percutaneous Sheath Introducer to permit venous access and catheter introduction into the central circulation.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
ChloraPrep and Hi-Lite Orange are trademarks or registered trademarks of Carefusion 2200, Inc.
SafetyGlide is a trademark or registered trademark of Becton, Dickinson and Company.
Teleflex, the Teleflex logo and Arrow are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-005075