Resources
Customer Support
Latin America /
Product Areas /
Interventional Cardiology/Radiology /
Cardiac Assist /
Intra Aortic Balloon Catheters

Intra-Aortic Balloon Catheters

Arrow® FiberOptix® Sensor Technology
The world's first fiber-optic IAB catheter.
- Designed to capture and transmit the high-fidelity AP signal at the speed of light
- Backup safety for AP signal: If required, the FiberOptix® Technology can be used as a traditional fluid-filled catheter
- Abrasion-resistant: The Cardiothane® II Membrane offers a unique design of abrasion resistant material with hydrophilic coating
- Universal design: Sheathed or sheathless insertion options; optional hemostasis device for post-insertion bleeding control
- Works with unique components of the AutoCAT2WAVE® and AC3 Optimus™ IABPs to deliver ProActive CounterPulsation® Technology
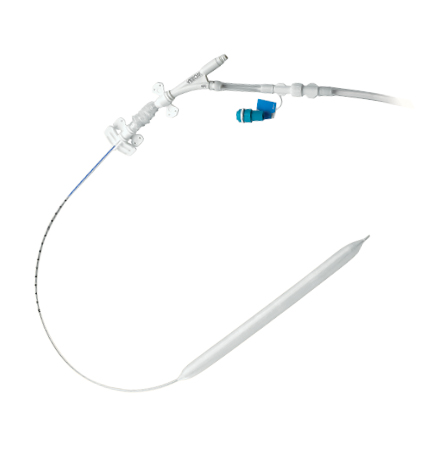
Fluid-Filled Catheters
UltraFlex™ 7.5 Catheter
- Kink-resistant, wire-reinforced catheter body
- The smallest wire-reinforced 30 and 40 cc IAB catheter currently available
- Designed to maximize arterial blood flow and help reduce limb ischemia due to 7.5 Fr. catheter size
Ultra 8® Catheter
- Fits through 8 Fr. sheath
- Large 0.027” central lumen design
- Soft, atraumatic blue bumper tip
RediGuard® Catheter
- Flexible, soft catheter design
- Smallest IAB catheter currently available - 7 Fr. 30 cc
- Wide range of IAB catheters from 30 to 50 cc
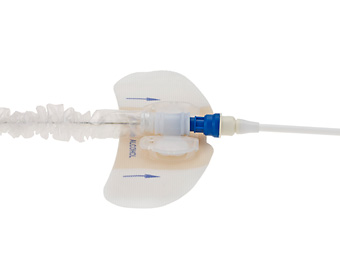
StatLock® Stabilization Device
StatLock® Device for Arrow® IAB Catheters is:
- Designed to remove the possibility of healthcare provider needlestick
- Designed to reduce suture-wound complications including occurrence of catheter-related bloodstream infections
- Designed to provide patient comfort
- Designed to provide an easier and faster alternative to suturing
The Arrow® IAB is utilized for
intra-aortic balloon
counterpulsation therapy in the aorta, whereby balloon inflation, during
diastole and deflation,
during systole increases blood supply to the heart muscle and decreases
work of the left
ventricle.
The StatLock® Device is a stabilization device for Arrow Interventional adult IAB catheters only.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
StatLock is a trademark or registered trademark of C.R. Bard, Inc.
Teleflex, the Teleflex logo, Arrow, AC3 Optimus, AERO, AutoCAT, AutoCAT2WAVE, AutoPilot, Cardiothane, FiberOptix, FlexLearning, and WAVE are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-005075
The StatLock® Device is a stabilization device for Arrow Interventional adult IAB catheters only.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
StatLock is a trademark or registered trademark of C.R. Bard, Inc.
Teleflex, the Teleflex logo, Arrow, AC3 Optimus, AERO, AutoCAT, AutoCAT2WAVE, AutoPilot, Cardiothane, FiberOptix, FlexLearning, and WAVE are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-005075