Arrow® OnControl® Powered Bone Marrow Biopsy System
Raising the Standard of Bone Marrow Biopsies
For blood-borne cancer patients, such as those with leukemia, lymphoma and multiple myeloma, and their treating oncologists, the bone marrow biopsy is a critical, but often painful procedure for diagnosis and treatment monitoring. In addition, when performed manually, the procedure is often physically taxing on clinical staff who must obtain a specimen of adequate size and quality. Ultimately, the diagnosis you receive from pathology is directly related to the integrity of this specimen.

That’s why the Arrow® OnControl® Powered Bone Marrow Biopsy System has become the preferred method of obtaining bone marrow specimens at major cancer centers. With the Arrow® OnControl® Powered Bone Access System, you can be confident in developing treatment plans based on the diagnosis you receive.
Bone marrow biopsies are widely regarded as being one of the more painful
procedures experienced
by patients. The resulting high level of anxiety, coupled with the
common disappointment of
inconclusive specimen results make many patients apprehensive about
scheduling follow-up
procedures. It’s these follow-up procedures that can make or break
effective treatment monitoring
for patients with leukemia, lymphoma, multiple myeloma
and other blood-borne
cancers. The Arrow® OnControl® Powered
Bone Marrow Biopsy System
helps alleviate that patient apprehension by providing results
faster1, and with less
pain than the manual alternative3.

Performing the biopsy or aspiration
Significantly less patient pain during and after the procedure. 1, 3, 5 Patients with leukemia, lymphoma, multiple myeloma and other blood-borne cancer patients frequently describe the bone marrow biopsy and aspiration as the “worst part of their treatment.” The Arrow® OnControl® Powered Bone Marrow Biopsy System helps significantly reduce pain by providing a faster procedure and reducing the need for “second attempts” that often occur when a specimen is of insufficient size or fails to capture.5
- Over 100% increase in the number of patients with little to no pain after 24 hours.1
- Patented threaded cannula design captures and holds core specimens from soft bone
- Increased user control and reduced physical requirements to obtain specimens, even with hard bone
- Reduced insertion pain5, and less overall pain3
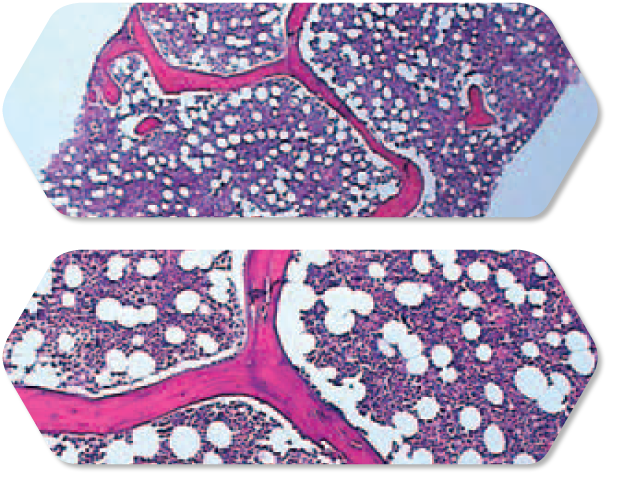
Analyzing specimens
Consistently larger, high quality core specimens.5
In most pathology departments, the bone marrow and aspirate specimens received vary widely, depending on the size of the patient, their bone hardness, and the experience or physical strength of the clinician. The Arrow® OnControl® Powered Bone Marrow Biopsy System effectively levels the field between clinicians, to help clinicians obtain consistently larger core specimens.
- More consistent, high quality specimens5
- More usable area for diagnosis5
- Longer, wider, and larger volume specimens3

Diagnosing and monitoring
Easier, more accurate diagnosis5 and treatment monitoring.
Larger, better quality specimens provide pathologists the material needed to make an accurate diagnosis, minimizing the inconvenience, delay, and pain associated with having to obtain another specimen. Physicians can be confident in developing treatment plans based on a diagnosis with a quality specimen for patients with blood-borne cancers and benign bone tumors.
- Greater overall patient satisfaction4
- Fewer second-attempt procedures required3 *
- Reduced pain during procedure helps promote patient compliance with ongoing or future testing3
Procedure scheduling
Procedures with increased efficiency.
When compared to biopsies performed with manual needles, the Arrow® OnControl® Powered Bone Marrow Biopsy System provides improved specimen quality5 and shortened procedure time1, that may help clinicians perform a greater number of biopsies per day for patients with blood-borne cancers and benign tumors.
- Fewer second-attempt procedures required3 *
- 55% faster procedure time to improve efficiency5 *
- Easy for clinicians to use, regardless of physical strength
* Compared to manual bone marrow biopsy procedures.
References:
1. Berenson JR, Yellin O, Blumenstein B, et al. Using a powered bone marrow biopsy system results in shorter procedures, causes less residual pain to adult patients, and yields larger specimens. Diagnostic Pathology 2011;6:23.*
3. Miller LJ, Philbeck TE, Montez DF, et al. Powered bone marrow biopsy procedures produce larger core specimens, with less pain, in less time than with standard manual devices. Hematology Reports 2011;3(e8):22-5. doi:10.4081/hr.2011.e8.*
4. Reed LJ, Raghupathy R,
5. Swords RT, Anguita J, Higgins RA, et al. A prospective randomized study of a rotary powered device (OnControl) for bone marrow aspiration and biopsy. J Clin Pathol 2011; 64(9):809-13. doi:10.1136/jclinpath-2011-200047.*
* Research sponsored by Teleflex Incorporated and its affiliates, including Vidacare LLC.