Resources
Customer Support - India
Teleflex Medical India Corporate Office
Teleflex Medical Private Limited
M/s Smartworks Coworking Space, Golden Millenium,
1st Floor 69/3, 44 Millers Rd, Vasanth Nagar, Bengaluru, Karnataka - 560052, India
Office Landline: +91 8040934790
Email Teleflex Medical India
Peripherally Inserted Central Catheter (PICC)
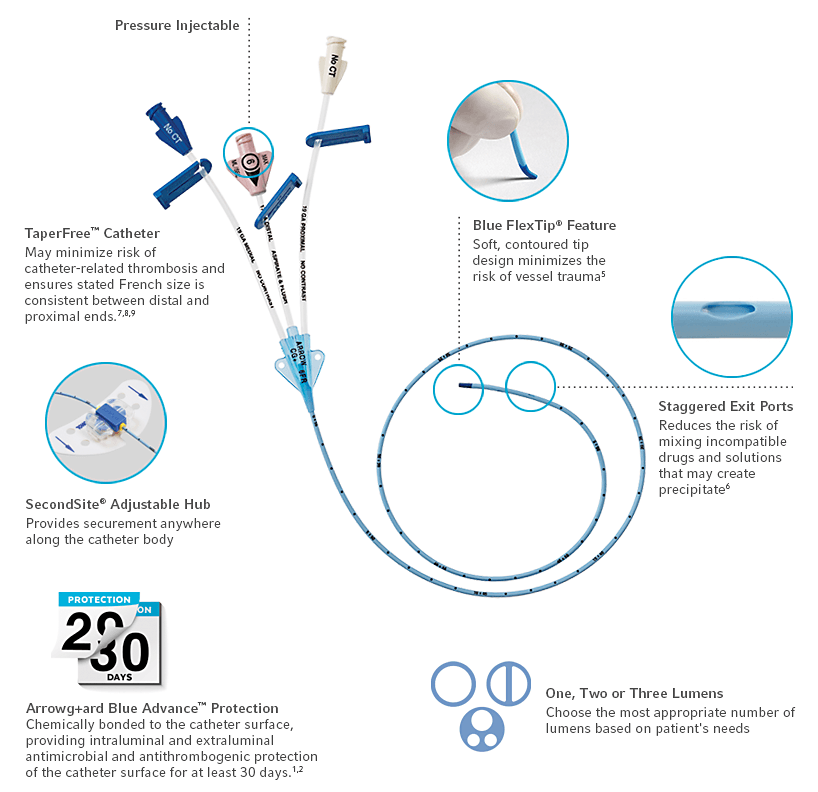
Additionally to the CVC products, we offer a range of PICCs in different set configurations. Designed and inspired by the same obsession that made our central venous catheters the industry leader – with features like e.g. the Arrow BlueFlex Tip.
Arrowg+ard Blue Advance PICC (Coming Soon)
The PICC with Antimicrobial and Antithrombogenic Protection1,2
The Arrowg+ard Blue Advance PICC is designed to protect both internal and external catheter surfaces with chlorhexidine, reducing the risk of these catheter-related complications:
- Intraluminal thrombotic occlusion2,3
- Microbial colonization1
- Phlebitis4
- Intimal hyperplasia4

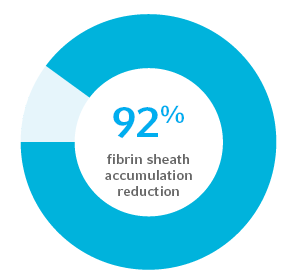
Demonstrated a 92% reduction of fibrin sheath accumulation on catheter surfaces at 31 days2
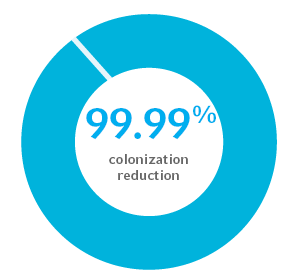
99.99% colonization reduction in gram-positive and gram-negative bacterial and fungal pathogens for at least 30 days1
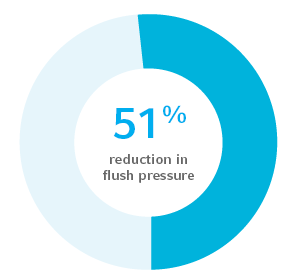
Reduced thrombotic intraluminal occlusion as evidenced by 51% reduction in flush pressure to clear thrombus3
Arrowg+ard Blue Advance PICC vs. Unprotected PICCs
In an intravascular in vivo model, Arrowg+ard Blue Advance Protection demonstrated a total of 92% reduction in fibrin sheath when challenged with Staph aureus, as compared to an uncoated PICC control. The Arrowg+ard Blue Advance PICC survived until the end of the study (mean 31 days) with no clinical signs of infection.2

Unprotected PICC Control Catheter
Day 5: Highly infected tissue and significant thrombus formation.

Arrowg+ard Blue Advance PICC
Day 31: Minimal thrombus formation and absence of microbial colonization.*
References:
-
In vitro data on file 2010: AVER-004371 and AVER-004483. No correlation between in vitro/in vivo testing methods and clinical outcomes have currently been ascertained.
-
As compared to uncoated PICCs, intravascular ovine model inoculated with Staph aureus: AVAR-000427. No correlation between in vitro/in vivo testing methods and clinical outcomes have currently been ascertained.
-
As compared to uncoated PICCs, in vitro model measuring flush pressure post exposure to bovine blood: AVER-006376. No correlation between in vitro/in vivo testing methods and clinical outcomes have currently been ascertained.
-
As compared to uncoated PICCs, intravascular ovine model: VAR-000427. No correlation between in vitro/in vivo testing methods and clinical outcomes have currently been ascertained.
-
Rosenbauer KA, Herzer JA. Surface morphology and tensile force at breaking point or different kinds of intravenous catheters before and after usage. Scan Electron Microsc. 1981;(Pt3):125-30.
-
Collins JL, Lutz RJ. In vitro Study of Simultaneous Infusion of Incompatible Drugs in Multilumen Catheters. Heart & Lung. 1991; 20(3):271-7.
-
Nifong TP and McDevitt TJ. The effect of catheter to vein ratio on blood flow rates in a simulated model of peripherally inserted central venous catheters. Chest 2011;140;48-53
-
Grove JR, Pevec WC. “Venous Thrombosis Related to Peripherally Inserted Central Catheters.” Journal of Vascular and Interventional Radiology. 2000; 11: 837-840
-
Trerotola, S, Stavropoulos, S, Mondschein, J, et al. Triple-lumen peripherally inserted central catheter in patients in the critical care unit: prospective evaluation. Radiology 2010;256(1):312-330
Contraindication:
Clinical assessment of the patient must be completed to ensure no contraindications exist. The Arrowg+ard Blue Advance PICC is contraindicated in the following areas:
• Patients with known hypersensitivity to chlorhexidine
• In presence of device related infections
• In presence of previous or current thrombosis in the intended vessel or along the catheterized vessel pathway.
No correlation between in vitro/in vivo testing methods and clinical outcomes have currently been ascertained.
Teleflex, the Teleflex logo, Arrow, Arrowg+ard Blue Advance, Blue FlexTip, SecondSite, and TaperFree are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. All other trademarks are trademarks of their respective owners. MCI-2020-0490