Indicated for use in patients with atherosclerotic disease of the femoral and proximal popliteal arteries, in particular for the treatment of insufficient results after Percutaneous Transluminal Angioplasty (PTA), e.g. residual stenosis and dissection.* *
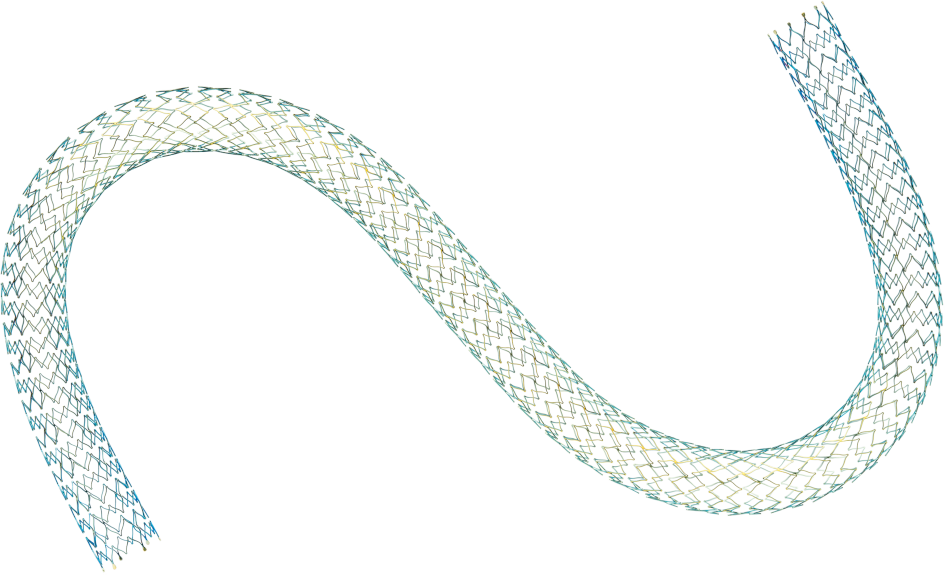
Product Highlights
140 μm thin struts
Pulsar-35 struts are thinner than the leading brands.1
Clinically Proven¤2,3
Long term safety and efficacy (24-month data), even in calcified lesions4,5
Tri-axial delivery system
Accurate stent deployment6
| Balloon catheter | |
|---|---|
| Catheter type | OTW |
| Recommended guide wire | 0.035” |
| Stent material | Nitinol |
| Strut thickness | 140 μm |
| Strut width | 85 μm |
| Stent coating | proBIO™ (Amorphous Silicon Carbide) |
| Stent markers | 6 gold markers each end |
| Sizes | ø 5.0 - 7.0 mm; L: 30 - 200 mm |
| Proximal shaft | 6F, hydrophobic coating |
| Usable length | 90 and 135 cm |
| Stent ø (mm) |
Catheter length 90 cm Stent length (mm) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6F | 30 | 40 | 60 | 80 | 100 | 120 | 150 | 170 | 200 | |||||
| 5 | 379878 | 379879 | 379880 | 379881 | 379917 | 379918 | 379919 | 379920 | 379921 | |||||
| 6 | 379883 | 379884 | 379885 | 379886 | 379922 | 379923 | 379924 | 379925 | 379926 | |||||
| 7 | 379888 | 379889 | 379890 | 379891 | 379927 | 379928 | 379929 | 379930 | 379931 | |||||
| Stent ø (mm) |
Catheter length 135 cm Stent length (mm) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6F | 30 | 40 | 60 | 80 | 100 | 120 | 150 | 170 | 200 | |||||
| 5 | 379898 | 379899 | 379900 | 379901 | 379937 | 379938 | 379939 | 379940 | 379941 | |||||
| 6 | 379903 | 379904 | 379905 | 379906 | 379942 | 379943 | 379944 | 379945 | 379946 | |||||
| 7 | 379908 | 379909 | 379910 | 379911 | 379947 | 379948 | 379949 | 379950 | 379951 | |||||